Hair-loss drug under lens over threat to infants, eye disorders | India News

NEW DELHI: Minoxidil, a widely used over-the-counter drug to treat hair loss, has been linked to unintended exposure in infants and a large number of eye-related side effects, according to a global safety analysis that has flagged dozens of baby-related cases and more than 1,600 eye disorder reports worldwide.Researchers identified more than 45 reports of excessive hair growth in infants aged up to 23 months linked to exposure to minoxidil. In several cases, exposure was not due to direct application but occurred unintentionally within the household, including contact with caregivers or contaminated surfaces.Globally, the analysis found 2,664 suspected cases of excessive hair growth linked to minoxidil use during pregnancy. Among infant cases, maternal exposure accounted for 22.2%, accidental exposure for 44.4%, while the route of exposure was unclear in 33.3% of cases. Though no infant cases were reported from India, the authors of the study cautioned that this likely reflected under-reporting rather than absence of risk, given the drug’s widespread use.The study also raised concerns about eye health. A total of 1,660 reports of eye-related adverse effects associated with minoxidil use were recorded globally, including 25 reports from India. Frequently reported problems included eyelid swelling and blurred vision.
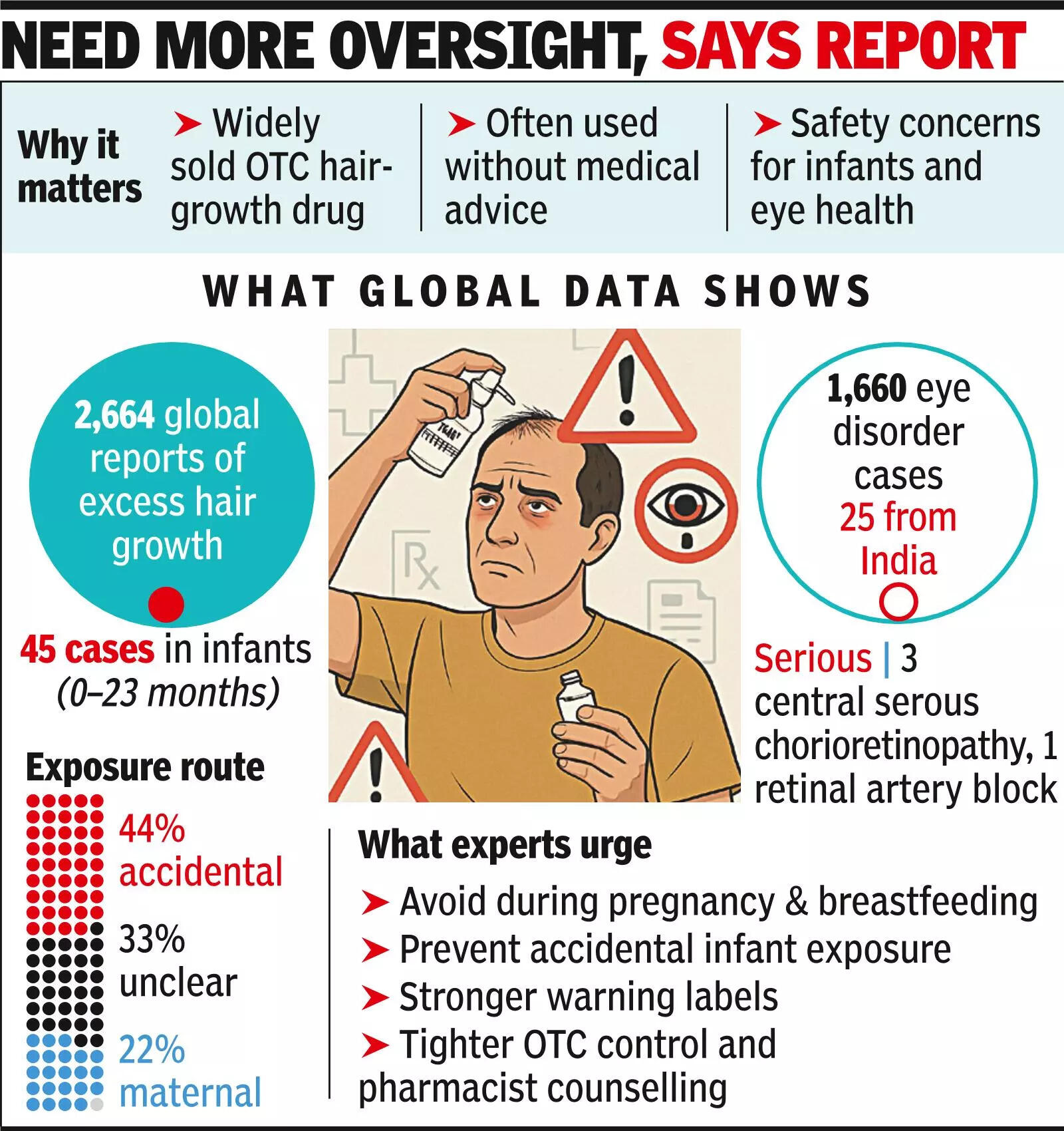
More serious eye conditions were also noted, including central serous chorioretinopathy. After removal of duplicate reports, four serious eye cases remained – three involving central serous chorioretinopathy and one case of retinal artery occlusion.The findings are based on a post-marketing safety analysis of adverse drug reports submitted to VigiBase, the global drug safety database maintained by WHO, covering data up to March 2025.The study was authored by Dr V Kalaiselvan and Dr Jaishree Suresh of the Indian Pharmacopoeia Commission, health ministry, and Dr Rohit Saxena of the Dr Rajendra Prasad Centre for Ophthalmic Sciences, AIIMS.Minoxidil was originally developed as an oral medicine to treat high blood pressure. Hair growth was later observed as a side effect, leading to its reformulation as a topical drug used to treat hair loss. Its effectiveness against androgenetic alopecia has since made it one of the most commonly used treatments worldwide.The authors warned easy over-the-counter availability, aggressive online promotion and limited counselling had allowed use to expand faster than safety awareness, particularly among pregnant women and households with infants.The study underlined that minoxidil was contraindicated during pregnancy and breastfeeding, but awareness of this warning remained low. With topical formulations commonly used at home, unintentional exposure through skin contact or household surfaces may place infants at risk.Calling for action, the authors recommended clearer warning labels, stricter oversight of over-the-counter sales and routine counselling by pharmacists and healthcare providers. Caregivers were advised to store products safely and avoid use around infants and pregnant women.While minoxidil continues to benefit adults using it to treat hair loss, the authors stressed public awareness and pharmacovigilance must keep pace with growing use to prevent avoidable adverse effects.






